Struggling to efficiently remove ethanol after extraction? Slow recovery processes and potential damage to your valuable compounds can be frustrating and costly. There must be a better, gentler way.
A rotary evaporator, or rotovap, is your best solution. It gently and quickly removes ethanol under vacuum. This protects your heat-sensitive extracts, speeds up your workflow, and allows you to reclaim and reuse your expensive ethanol, saving both time and money in the long run.

I've spent over 16 years in this industry, helping people set up labs all over the world. A common problem I see is the bottleneck caused by solvent removal. You have this beautiful, potent extract, but it's swimming in ethanol. Getting that ethanol out without destroying the product is the real challenge. The right equipment doesn't just make the job possible; it makes it profitable and consistent. That's where I believe our experience really helps our customers succeed.
What Exactly is a Rotary Evaporator and Why Use It for Ethanol?
You have a solution of ethanol and extract, but need to separate them. Traditional heating methods are too harsh and can easily degrade your final product, wasting your hard work.
A rotary evaporator is a specialized distillation tool. It works by lowering the boiling point of ethanol with a vacuum. Then, it rotates the flask to create a thin film, dramatically increasing the surface area for fast, low-temperature evaporation, preserving your extract's quality.
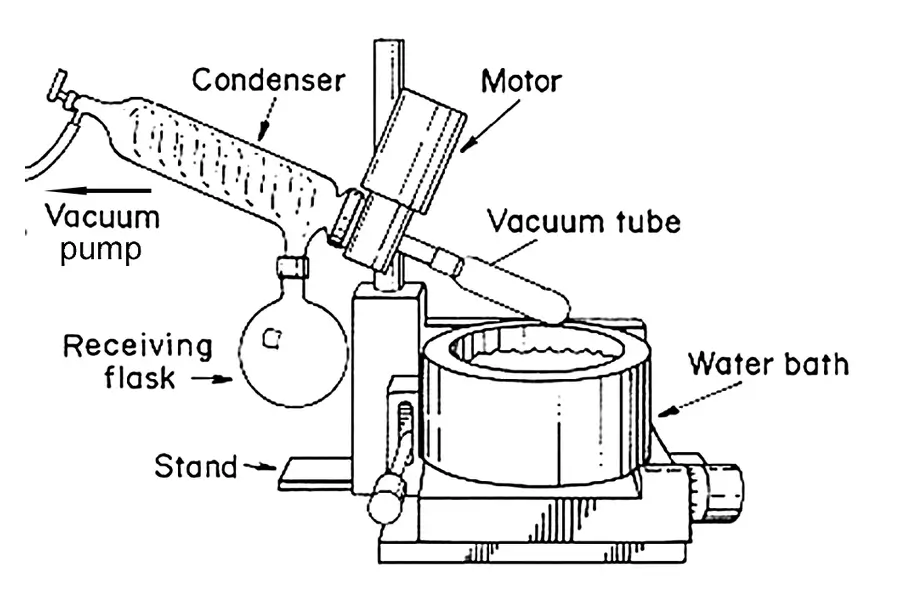
Let's break down why this is the go-to method. At standard atmospheric pressure, ethanol boils at about 78°C (173°F). Many valuable compounds in plant extracts, like terpenes or certain cannabinoids, are volatile and can be damaged or lost at that temperature. A rotary evaporator machine tackles this problem head-on. By applying a vacuum, we lower the pressure inside the system. This directly lowers the boiling point of ethanol, often to a much safer temperature around 40°C (104°F) or even lower. The rotation of the flask is just as important. Instead of boiling a static pool of liquid, the rotation spreads the solution into a thin, constantly renewed film on the inner surface of the glass. This massively increases the evaporation efficiency. It's the difference between drying a puddle and drying a thin layer of water wiped across a table. This combination of low temperature and high efficiency makes it perfect for preserving the delicate profile of your extracts.
How Do You Set Up a Rotary Evaporator for Optimal Ethanol Recovery?
Setting up lab equipment can seem complex. Connecting the wrong parts or using incorrect settings can lead to poor results, equipment damage, or even safety hazards in your lab.
For optimal setup, connect the vacuum pump to the condenser, place the rotating flask in the water bath, and ensure the receiving flask is secure. Set the water bath to 40-50°C, the condenser chiller to 0°C or below, and gradually apply the vacuum.

Getting the setup right is critical for success. I always walk my customers through this. First, make sure all your glassware is clean and has no cracks. A tiny crack can become a big problem under vacuum. Attach your round-bottom flask, filled no more than halfway with your ethanol mixture, to the rotating motor. Use a joint clip to secure it. Connect the condenser to a chilling unit; for ethanol, you want the coolant temperature to be very low, ideally 0°C or even -10°C, to ensure all the evaporated ethanol vapor turns back into a liquid. Then, connect a vacuum pump with a cold trap in between. The cold trap protects your pump from solvent vapors. Finally, lower the rotating flask into the water bath. A good starting point for your main settings would be:
Key Setup Parameters
Water Bath Temperature: 40°C. This provides enough energy for evaporation without being too hot.
Rotation Speed: Start around 100-120 RPM. This creates a good film without excessive splashing.
Chiller Temperature: 0°C or lower. This is crucial for efficient condensation and recovery of your ethanol.
Vacuum Level: Gradually apply vacuum. Watch for bumping (violent boiling). You can adjust the vacuum to control the boil rate. A 5L rotary evaporator is a great starting point for scaling up from the benchtop.
What Factors Influence the Efficiency of Ethanol Evaporation?
You're running your rotovap, but the process is slow. The ethanol isn't evaporating as quickly as you hoped, creating a frustrating bottleneck in your production workflow and delaying results.
Efficiency hinges on four key factors: the temperature difference between the heating bath and the solvent's boiling point, the vacuum level, the flask's rotation speed, and the size and capacity of the condenser. Optimizing these parameters is essential for speed and success.

I often get asked how to speed things up. It's a balancing act. You need to find the sweet spot for your specific setup. Increasing the bath temperature or the rotation speed will generally increase the evaporation rate, but only up to a point. If you go too high, you risk bumping or degrading your product. The most significant factor is often the vacuum depth. A deeper vacuum (lower pressure) means a lower boiling point, which creates a larger temperature difference (Delta-T) between your bath and your boiling solvent, driving faster evaporation. However, your vacuum pump and condenser must be able to handle the increased vapor load. If your condenser can't re-condense the vapor as fast as it's created, you'll lose solvent and efficiency. This is particularly important with a large scale rotary evaporator, where the volume of vapor is substantial. Here's a simple table to illustrate the relationship:
| Parameter | Effect on Efficiency | Consideration |
|---|---|---|
| Bath Temperature | Higher temp = faster evaporation | Risk of product degradation if too high. |
| Rotation Speed | Higher speed = larger surface area | Risk of splashing if too fast. |
| Vacuum Level | Deeper vacuum = lower boiling point | Must not overwhelm the condenser. |
| Condenser Temp | Lower temp = better condensation | Ensures high recovery rate of ethanol. |
How Do You Choose the Right Rotary Evaporator for Your Needs?
Buying a rotovap feels like a huge decision. You see models of all sizes and prices, but you're not sure which one matches your batch size, your future plans, or your budget.
Choose your rotovap based on your typical batch volume. For R&D, a 2L rotary evaporator is ideal. For pilot scale or small production, consider a 10L or 20L model. For large-scale processing, a 50L or larger unit is necessary. Match the size to your workflow.

Over the years, I've helped thousands of clients select the right equipment. The most important question I ask is: "How much material do you need to process per day?" Your answer determines the size. Don't just think about today; think about where you want to be in a year. Buying a unit that's too small is a common mistake that quickly limits growth. On the other hand, buying a massive unit for tiny batches is inefficient. For a university or research lab doing initial discovery, a small, flexible laboratory rotary evaporator is perfect. If you're a business developing a product and running pilot batches, a 10L or 20L rotovap offers a great balance of capacity and footprint. For full commercial production, especially in the cannabis or hemp industry, you need a robust 50L or even a 100L machine to keep up with demand. Also consider the features: do you need an electric lift for the bath? Do you prefer digital controls for temperature and rotation? These quality-of-life features can make a huge difference in daily operation.
Conclusion
A rotary evaporator is an essential tool for high-quality ethanol extraction. Choosing the right size and using it correctly will boost your efficiency, protect your product, and save you money.



