How Does a Rotary Evaporator Revolutionize Laboratory Distillation?
Struggling with slow, inefficient solvent removal that risks degrading your samples? The traditional distillation process can be a major bottleneck in any lab, wasting both time and valuable compounds.
A rotary evaporator, or rotovap, gently and quickly removes solvents by reducing pressure, which lowers the solvent's boiling point. This process protects sensitive materials and dramatically speeds up evaporation, making it an essential tool for modern chemistry, pharma, and research labs.

As a manufacturer with over 16 years of experience, I've seen countless labs transform their workflows with this single piece of equipment. It's not just about speed; it's about gaining precise control over a delicate process. Moving from old methods to a rotovap feels like upgrading from a flip phone to a smartphone. It opens up possibilities and efficiencies you didn't know you were missing, and I'm here to walk you through how it all works.
What Are the Core Components of a Rotary Evaporator Machine?
Ever wondered what makes a rotovap so effective? It seems complex, but its design is a clever combination of a few key parts working together for one purpose: efficient evaporation.
The main components are a heated water or oil bath, a rotating evaporating flask, a vacuum system to lower pressure, and a condenser with a collection flask. Each part plays a critical role in ensuring gentle, rapid solvent removal under controlled conditions.
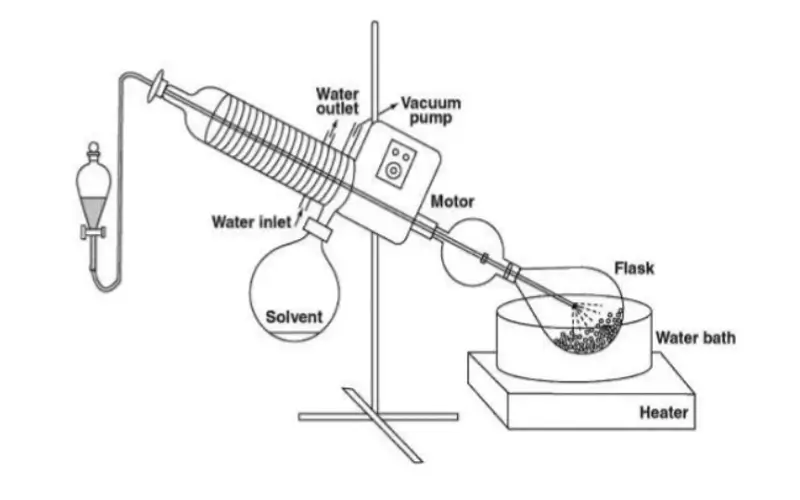
Understanding how these parts interact is key to mastering the instrument. I often tell my customers to think of it as a coordinated team. The vacuum pump is the foundation, creating a low-pressure environment. The heated bath provides the energy for evaporation, but gently. The rotation of the flask is the genius part; it constantly coats the inner surface with a thin film of the sample. This massively increases the surface area, which dramatically speeds up the evaporation process. Finally, the condenser acts as the capture unit, using a coolant to turn the solvent vapor back into a liquid, which drips neatly into the collection flask. This not only separates your solvent but also allows for its recovery and reuse.
Component Breakdown:
Heating Bath: Provides uniform heat. Water is used for boiling points under 100°C, while oil is used for higher temperatures.
Rotating Flask: This is where your sample goes. Rotation prevents bumping and increases the surface area for faster evaporation. Models like our popular 5L rotary evaporator are perfect for many standard lab tasks.
Vacuum System: The heart of the low-temperature distillation. It removes air, lowers the boiling point, and pulls the vapor through the system.
Condenser: A set of cooled coils (using water or another coolant) that turns the solvent vapor back into a liquid.
Collection Flask: Catches the distilled, purified solvent.
Why is Distillation Under Vacuum More Efficient?
Tired of damaging your heat-sensitive compounds with high temperatures? Applying heat is necessary for distillation, but too much can destroy the very thing you're trying to isolate.
Distilling under vacuum is highly efficient because it significantly lowers a solvent's boiling point. This means you can evaporate solvents at much lower, safer temperatures, protecting your sample from thermal degradation while speeding up the entire separation process.
I remember a client in the food and beverage industry who was struggling to extract delicate flavor compounds from botanicals. Using traditional atmospheric distillation, the high heat was "cooking" the extracts, ruining the final product's aroma. When we introduced them to a laboratory rotary evaporator, it was a game-changer. The ability to distill at 30-40°C preserved the fragile molecules perfectly. This principle is based on simple physics: a liquid boils when its vapor pressure equals the pressure of the surrounding environment. By using a vacuum pump to remove air, we lower the ambient pressure. Less pressure means the liquid needs less heat energy to start boiling. This method is not just safer for the product; it's also faster and requires less energy, making it a more sustainable and cost-effective choice for any lab.
Key Advantages of Vacuum Distillation:
| Advantage | Explanation | Typical Application |
|---|---|---|
| Lower Temperature | Prevents thermal decomposition of sensitive compounds. | Pharmaceuticals, natural products, flavor extracts. |
| Increased Speed | The combination of rotation, heat, and vacuum accelerates evaporation. | High-throughput labs, solvent recovery. |
| Higher Purity | Allows for sharper separation between solvents and solutes. | Purifying synthesized chemical compounds. |
How Do You Choose the Right Rotary Evaporator for Your Lab?
Feeling overwhelmed by the options? Selecting the wrong size or type of rotovap can lead to inefficiency, wasted space, and poor results, setting your research or production back.
To choose the right rotary evaporator, you must first assess your sample volume to determine the flask size (from 1L to 50L). Then, consider the types of solvents you use to select compatible seals and a proper vacuum pump. Finally, match the features to your workflow needs.

Over the years, I’ve helped thousands of customers make this exact decision. The most common mistake is focusing only on price. The best approach is to start with your application. Are you a university lab doing varied research? A flexible 2L rotary evaporator might be perfect. Are you a cannabis processor handling large batches of ethanol extract? You’ll need a robust, large scale rotary evaporator with a powerful chiller and pump. We manufacture a full range, from small benchtop units to massive 50L systems, because we know one size doesn't fit all. Thinking about your future needs is also important. If you expect your research or production to scale up, investing in a slightly larger system now can save you a lot of money and hassle down the road. It's about finding the right balance between capacity, features, and budget.
Decision Checklist:
Capacity: How much sample will you process at once? A university lab might use a 2L or 5L model, while industrial production might require a 20L or 50L rotary evaporator.
Solvent Type: What chemicals will you use? This determines the necessary seal material (e.g., PTFE for aggressive chemicals) and the type of vacuum pump.
Automation: Do you need a manual lift or an automated one? Automated lifts add safety and convenience, especially for larger flasks.
Safety Features: Look for coated glassware for protection and over-temperature shut-off features in the heating bath.
Conclusion
A rotary evaporator is an indispensable tool for safe, efficient distillation. Choosing the right one transforms your lab's capability, protecting samples and accelerating discovery.

E-mail:
WhatsApp:
Address:
19/F, Block B, Guohong Mansion, Hi-Tech Development Zone, Zhengzhou City, Henan Province, China
Related blogs
You May also like
Rotary Evaporator Machine - Complete Guide
Comprehensive guide to rotary evaporator machines covering working principles, components, applications, technical specs, and tips for selecting the right mod...
Read MoreExplosion Proof Rotary Evaporator - Your Lab's Safety Guardian
Discover why explosion proof rotary evaporators are essential lab safety equipment. Learn how they prevent solvent fires, save costs long-term, and explore mo...
Read MoreEthanol Rotary Evaporator Guide
Discover how ethanol rotary evaporators work, their cost-saving benefits, and how to choose the right model. Learn solvent recovery techniques, safety practic...
Read More


